- Contact Us
- OCTANE
- CGP@uhn.ca
Significant progress has been made in the treatment of cancer through the use of targeted drugs, but what works for one patient may not work for another. Cancer cells typically have changes in their genes that make them different from normal cells, and targeted drugs take advantage of these differences to target the cancer cells. Biomarkers are specific characteristics of cancer cells, such as mutations in their DNA, that help doctors identify which drug treatments are likely to be effective or how likely a cancer is to spread.
patients have been enrolled on OCTANE
patients with completed NGS (profiling) of tumors to date
tumor tissues samples collected for future research
blood samples collected for future research
patients with actionable mutations received matched targeted therapy through OCTANE
OCTANE's mission is to expand the capacity of next-generation sequencing (NGS) testing for advanced solid tumor patients across Ontario while creating a repository (storage) of blood and tumor samples from patients which can be used for future research.
Patients help advance research by providing us access to their relevant clinical data and biological samples. The OCTANE team analyzes and de-identifies the data (i.e. removes identifiers like name, address, phone number) before sharing it through scientific data-sharing projects to help improve our understanding of cancer.
In 2016, the Cancer Genomics Program (CGP) launched the Ontario-wide Targeted Nucleic Acid Evaluation (OCTANE) study, an initiative that is jointly supported by Princess Margaret Cancer Foundation (PMCF) and the Ontario Institute for Cancer Research (OICR). Thanks to the financial support from PMCF and OICR, CGP was able to establish the core infrastructure for OCTANE including a central study management team, a central biobank at OICR for storing samples to be used in future research, and a Steering Committee that includes clinical and laboratory scientists from all participating institutions across Ontario.


Celeste Yu
Cancer Genomics Program (CGP) Manager
Princess Margaret Cancer Centre
Samanta Del Rossi
Sponsor Coordinator
Princess Margaret Cancer Centre
Patient Partners
OICR Patient and Family Advisory Council (PFAC)
The OCTANE team gratefully acknowledges the support from the Government of Ontario (through the Ontario Institute for Cancer Research Adaptive Oncology), and the Princess Margaret Cancer Foundation (through the Cancer Genomics Program at University Health Network).


If you are about to start treatment for your solid cancer, *, please speak to your medical oncologist. Your medical oncologist will assess you to determine if you meet the eligibility criteria for either OCTANE 1.0 or OCTANE 2.0.
*Blood cancers such as leukemia, lymphoma and myeloma are not eligible to participate in OCTANE.
If you are eligible to participate, you will be presented with a consent document at your clinic appointment. Take the time to read it. A verbal explanation of the consent document will also be provided. Talk to your doctor, family, and/or friends about the risks and benefits of taking part in the study. All of your questions regarding participation in the study will be answered. It is important that you have as much information as you need and that all of your questions are answered.
If you are participating in OCTANE 1.0, you will be asked to provide two tubes of blood (about 20 mL or 4 teaspoons) when you are at the hospital during one of your routine bloodwork appointments. This blood sample will be stored for future research.
If you are participating in OCTANE 2.0, you will be asked to provide four tubes of blood (about 40 mL, or 3 tablespoons), at up to 6 time points when you are already at the hospital for your routine bloodwork. These blood samples will be stored for future research or testing, which may include ctDNA testing (looking at small fragments of DNA from cancer cells that are shed into the bloodstream).
OCTANE 1.0: Some of the leftover tissue from your prior surgery or biopsy (archival tumor tissue) will be sent to your institution's laboratory for gene sequencing. Around 8 weeks later, you and your study doctor will get the results of this sequencing.
OCTANE 2.0: Your archival tissue sample will be requested for future genomic analysis that may include whole genome sequencing. Whole genome sequencing refers to the process of determining your entire DNA sequence. You will not receive the results of this testing.
If your cancer progresses or comes back, you may be asked to have an optional biopsy collected for the study. This biopsy may be done as part of clinical care. The study biopsy takes small pieces of tumor tissue from your body. These samples will be sent to the Advanced Molecular Diagnostics Laboratory (AMDL) at the Princess Margaret Cancer Centre, where targeted gene sequencing (sequencing specific genes that are known to be linked to cancer) is performed. You and your study doctor will get the results of the testing performed on the biopsy sample.
The study team will collect information that is needed for the study from your medical health records. This information will include your cancer type, relevant past medical history, ongoing cancer treatments, CT/MRI images and reports and pathology information related to the samples being used in this study.
For both OCTANE 1.0 and 2.0, the results from gene sequencing will list the relevant gene changes (mutations) found in your cancer sample, as well as important biomarker information. Your doctor may use this information to guide treatment decisions. The results may be added to your medical records.
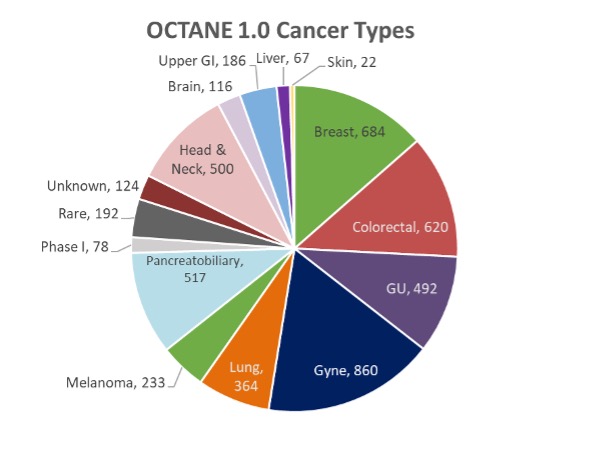
We are enrolling patients with advanced, incurable solid tumors. As of December 2022, we have enrolled more than 5000 patients with advanced solid tumors, with the largest group being gynecological cancers followed by breast, colorectal, and pancreatobiliary (pancreas, bile duct, gallbladder, ampullary) cancers.
The second stage of this study will enroll participants with specific types of cancer into two broad categories:
All cancers arise from changes that occur in the DNA of cells. DNA is the chemical set of instructions inside each cell that tells the cell what to do. The complete set of DNA within a species is called the genome. When the DNA instructions have mistakes in them, cells may not function normally and may grow out of control, causing cancer. The study of genes associated with cancer is referred to as cancer genomics. Cancer-causing changes in DNA, called mutations, can be inherited, but most are acquired throughout life. While cancer was once thought of as a single disease that affected many different parts of the body, researchers now know that there are differences in the DNA makeup of cancer cells of each patient and that changes in the cancer's DNA can cause each cancer to have a unique behaviour. That is why two patients who have cancer in the same part of the body may respond differently to the same treatment. Testing for mutations in cancer cells is known as molecular profiling.

Genomic changes in cancer can potentially provide information on the benefits or resistance to certain targeted cancer treatments. We can already see the success of targeted cancer treatments in various cancers, such as the use of imatinib in chronic myelogenous leukemia (CML); trastuzumab in human epidermal growth factor receptor-2 (HER2) amplified breast cancer; and epidermal growth factor receptor (EGFR) inhibitors in lung cancers.
By looking at the molecular profile of many tumor samples from many different patients, we will be able to gain a better understanding of what makes one cancer different from another, which is important because it will help explain why two patients with the same type of cancer may respond very differently to the same treatment. By connecting specific genomic changes with specific outcomes, we will be able to develop more effective and individualized ways of treating each cancer patient.
To date, profiling results from 4200 patients have been successfully
completed as part of OCTANE 1.0. These results are uploaded to the
study's central database for sharing of de-identified data across
all of the participating hospital sites.
OCTANE data has been used in multiple scientific publications,
posters, abstracts and presentations. Here are a few examples:
PUBLICATIONS
OCTANE is an Ontario-wide initiative consisting of two study stages.
The first stage of OCTANE (OCTANE 1.0) is enrolling patients with advanced, incurable solid tumors. OCTANE 1.0 aims to expand next-generation sequencing (NGS) panel testing of tumor tissue for cancer patients across Ontario while building a rich biobank (storage of tumor tissue, blood and other biological samples) for future research. Archival tumor samples (previously collected biopsy or surgical tumor samples) will be tested to provide biomarker data about a patient's cancer. This information may help physicians in guiding the use of approved treatments as well as identifying which clinical trials of new drug treatments may be most appropriate for the patient in the future.
This study will be used to develop a province-wide registry of targeted gene sequencing testing that will be made available to cancer researchers. Additional tumor tissue and blood samples collected from study participants will be stored in a biobank for future research.
The second stage of OCTANE (OCTANE 2.0) builds on OCTANE 1.0 and is enrolling participants with specific types of cancer, (such as cutaneous melanoma, lung cancer, sarcoma, etc) who are about to start specific forms of treatment. OCTANE 2.0 focuses on the research of two big issues: 1) why cancer returns (relapses) and 2) how it stops responding to a treatment that may have worked in the past. Molecular residual disease (or MRD) refers to small amounts of cancer cells that remain in the body after surgery to remove the primary tumor. MRD can be detected with sensitive laboratory techniques that measure small fragments of DNA shed by cancer cells into the bloodstream (circulating tumor DNA, or ctDNA). Computer models that are based on information from molecular residual disease (MRD) and imaging tests (looking at the shape, size and texture of the tumor on CT or MRI images) will be used to help predict which treatments will be helpful and find patients who are at high risk of having their cancer return. Blood samples will be collected during treatment and follow-up along with CT or MRI scan images that doctors use to monitor treatment response and identify when cancer recurs. The ctDNA levels from the blood samples will be tracked and computer-based models will combine these results with imaging scans to build tools that can help doctors identify MRD and provide a window to intervene earlier with treatments before relapse occurs.
The medical oncologists participating in the OCTANE study will assess potential participants to determine which stage of the study is suitable.
There are both risks and benefits to taking part in this study.
General Risks
This study will use a sample of your tissue. Generally, your hospital will keep some of your tissue. This tissue may be used to help treat your cancer in the future. Because this study will need to use some of this tissue, there is a small risk that it could be used up.
Genetic Testing Risks
When you donate your blood or tissue for genetic testing or research, you are sharing genetic information not only about yourself, but also about biological (blood) relatives who share your genes or DNA. There is a risk that information gained from genetic research could eventually be linked to you. The study doctors believe the risk of this happening is very small. However, the risk may increase in the future as people find new ways of tracing information. The study team has procedures and security measures in place to ensure it will be extremely difficult for this to happen. Each patient sample in this study will be uniquely identified by a study ID (study code) and all samples will also be tracked by a barcode. Samples will be held in a secure facility and your samples will not be identified by name.
Blood Sample Collection Risks
Common side effects of blood collection are the potential for bleeding, bruising, discomfort, infection or pain at the needle site, or dizziness from the needle stick to take the blood samples.
Biopsy Risks
Possible side effects of a biopsy are a small amount of bleeding at the time of the procedure, bruising, swelling or pain at the biopsy site, dizziness or fainting and fever. Pain can be treated with regular pain medications. Rarely, an infection can occur.
Personal Health Information Risks
There is a risk that someone could get access to the personal information in your medical records or other information researchers have stored about you.
There is a risk that someone could trace the information in a central database back to you. Even without your name or other identifiers, your genetic information is unique to you. The researchers believe the chance that someone will identify you is very small, but the risk may change in the future as people come up with new ways of tracing information.
Due to the rapid pace of technological advances, the potential future use of genetic information is unknown and therefore the potential future risks also are unknown.
Benefits
There may be no direct benefit to you from being in this study. The results may or may not help your treating oncologist determine what treatment to recommend to you for your cancer. This study may help the study doctors learn things that may help other people in the future.
You will not be paid for taking part in this study.
There are no costs to take part in this study. You will not have to make any extra trips to the hospital. You will not have to pay for any of the tests performed on your samples.
After removing all personal information that could identify you, some of your health information (such as your targeted gene sequencing results, age, cancer type and pathology information, response to cancer treatment, and medicines you took) will be kept by the Princess Margaret Cancer Centre in a central research database or shared with collaborating researchers outside of this hospital. If information from this study is published or presented at scientific meetings, your name and other personal information will not be used.
If you consent to OCTANE, your blood samples may be used to provide a source of DNA from your normal (non-cancerous) cells that may be used for future research. Your blood samples will also be used to assess circulating tumor DNA (small fragments of DNA from cancer cells that are shed into the bloodstream).
If you consent to OCTANE 1.0, your tissue samples will be sent to your institution's laboratory for gene sequencing. Additional samples of your stored tumor tissue, beyond what is needed for gene sequencing, will be stored in a research tissue biobank at the Ontario Institute for Cancer Research for future research. You will not receive the results of this future research.
If you consent to OCTANE 2.0, some of the leftover tissue from your cancer surgery or biopsy will be requested for future gene sequencing. You will not receive the results of this testing.
If you consent to OCTANE 2.0, and your cancer gets worse or comes back, you may be asked to have a biopsy of your cancer for the study or as part of the care you are receiving for your cancer. The biopsy takes small pieces of cancer tissue from your body. These samples will be sent to the Advanced Molecular Diagnostics Laboratory (AMDL) at the Princess Margaret Cancer Centre for gene sequencing. You and your doctor will get the results of this testing.
You can decide to stop taking part in the study at any time, and it will not affect the usual care or treatment you are receiving for your cancer. If you decide to stop, let your study doctor know as soon as possible. If you stop, you can decide if you would like the study doctor to continue to contact you to find out how you are doing.
You may withdraw your permission to use your personal health information for this study at any time by letting the study doctor know. However, this would also mean that you withdraw from the study. Your study data that was recorded before you withdrew from the study will be used but no new information will be collected or sent to the sponsor after you withdraw your permission.
You may choose to have your samples destroyed at any time. If you no longer want your samples to be used in this research, you should tell your study doctor. Your study doctor will notify the sponsor, who will ensure the samples are returned to the hospital from which they were obtained if needed, or destroyed. If tests have already been done on your sample(s), it will not be possible to withdraw those results. However, no further testing will be done.
Your study doctor will tell you in a timely manner about new information or changes in the study that may affect your health or your willingness to continue in the study.
For general inquires, please email
CGP@uhn.ca
or visit our website:
http://www.cancergenomicsprogram.ca
For trial updates, please visit the ClinicalTrials.gov
website:
https://clinicaltrials.gov/ct2/show/NCT02906943
For more information about the Advanced Molecular Diagnostics Laboratory, the lab that performs NGS testing for OCTANE, please visit: https://amdl.uhnresearch.ca